Abstract
Introduction:
Chronic graft-versus-host disease (cGVHD) is a common complication post allogeneic hematopoietic cell transplant. The goal of cGVHD treatment is symptom relief and long-term control of disease progression, with minimal toxicity/adverse events. Available treatments, including corticosteroids, mTOR inhibitors, calcineurin inhibitors, extracorporeal photopheresis, mycophenolate mofetil, ibrutinib, and ruxolitinib, are associated with adverse events (AEs) that may limit compliance or lead to treatment discontinuation. Belumosudil is a selective Rho-associated coiled-coil kinase 2 (ROCK2) inhibitor that is approved for the treatment of cGVHD for adult and pediatric patients 12 years and older after failure of at least two prior lines of systemic therapy (i.e., 3L/4L+). Belumosudil has been well tolerated in clinical trials, with a low incidence of grade ≥3 AEs, including cytopenias and infections, which may lead to increased compliance to treatment and lower overall costs. The present analysis estimated the budget impact of increasing utilization of belumosudil in 3L/4L+ cGVHD in three scenarios using a United States (US) payer perspective.
Methods:
A 5-year budget impact model was developed to estimate annual healthcare resources utilization (HCRU) and budget impact for a 10 million member US payer plan. Epidemiology, market share, treatment cost, adverse events, and HCRU inputs were incorporated in the model in a user-modifiable manner. US cGVHD prevalence rates were incorporated based on literature and secondary sources (Bachier et al. 2021). In addition to belumosudil, treatments that can potentially be accounted for in the model include corticosteroids, calcineurin inhibitors, mTOR inhibitors, mycophenolate mofetil, extracorporeal photopheresis, ibrutinib, and ruxolitinib (off-label), with proportions based on current and projected market share. Belumosudil use was modeled in three different scenarios based on projections of branded agent shares (i.e., source of share from ibrutinib only, ruxolitinib only, or both ibrutinib and ruxolitinib in a market with branded agents only). Grade 3/4 adverse events and their rates in cGVHD were sourced from prescribing information, clinical trials, or published literature and were monetized using Medicare Diagnosis Related Groups costs, with conversion to commercial plan costs utilizing ratios from the Congressional Budget Office. Drug costs were sourced from Redbook. HCRU and costs per visit were derived from a prior cGVHD claims analysis. The overall percentage reduction and per member per month (PMPM) savings were calculated.
Results:
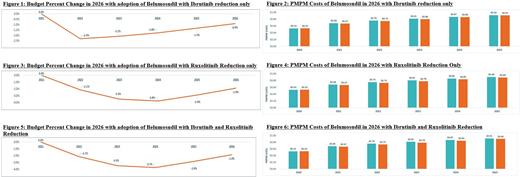
When belumosudil share is taken only from ibrutinib, by 2026, the model estimates a 0.9% reduction in the plan's budget and a PMPM savings of $0.01 versus a market without belumosudil. Percentage cost offsets due to reductions in AE and HCRU costs with belumosudil adoption were 34.3% and 13.5%, respectively. In a scenario where belumosudil share is taken only from ruxolitinib, an estimated reduction in budget of 1.9% and a savings of $0.01 PMPM would be observed by 2026, largely due to cost reductions of 76.2% and 27.9% in AEs and HCRU, respectively. If belumosudil share is derived from both ibrutinib and ruxolitinib similar results were observed, with a 1.9% reduction in budget and $0.01 in PMPM savings by 2026. AE and HCRU cost reductions versus a scenario without belumosudil were 71.2% and 27.7%, respectively.
Conclusions:
The introduction of belumosudil as a therapy for 3L/4L+ cGVHD patients resulted in a budget reduction in this model for US health plans. In each of the three scenarios examined, focusing on patients receiving existing branded agents in 3L/4L+ cGHVD, belumosudil demonstrated 0.9-1.9% in overall and $0.01 in PMPM savings, likely driven by the favorable safety profile of belumosudil reducing AEs and HCRU. The model indicates that belumosudil may provide an alternative to existing cGVHD treatments while also offering cost savings to a US health plan. Patients who enter 3L/4L+ cGVHD treatment may benefit from recently approved therapies for cGVHD like belumosudil that offer a well tolerated treatment option for patients who are cycling through cGVHD therapies.
Bachier: BMS: Membership on an entity's Board of Directors or advisory committees; CRISPR: Membership on an entity's Board of Directors or advisory committees; Autolus: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Mana: Membership on an entity's Board of Directors or advisory committees; Nkarta: Membership on an entity's Board of Directors or advisory committees. Skaar: Trinity Life Sciences: Current Employment; Kadmon Corporation: Consultancy. Dehipawala: Trinity Life Sciences: Current Employment; Kadmon Corporation: Consultancy. Miao: Trinity Life Sciences: Current Employment; Kadmon Corporation: Consultancy. Ieyoub: Kadmon Corporation: Current Employment. Taitel: Kadmon Corporation: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal